Uncharted Territory
Landmark stem cell trial offers hope to ALS patients
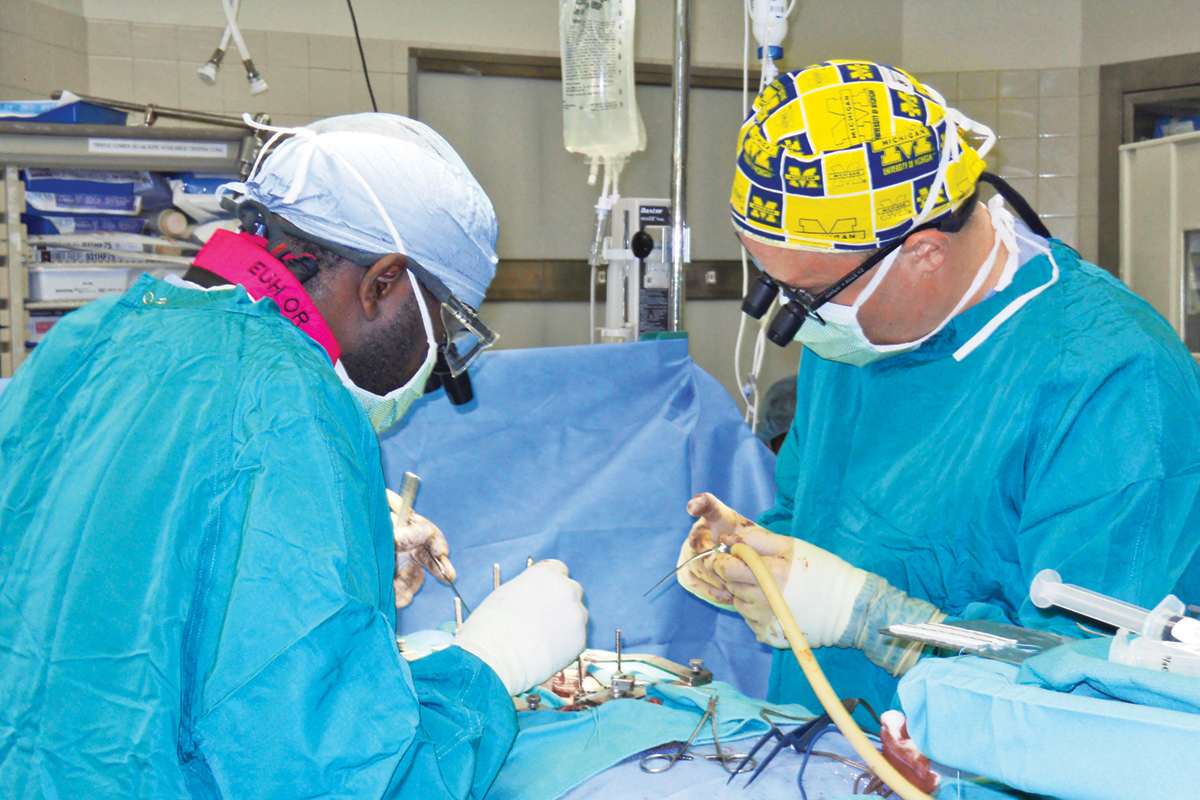
Courtesy Nicholas Boulis
About six months ago, on the eve of his fifty-ninth birthday, John Conley was preparing to let neurosurgeons at Emory University Hospital inject stem cells into his spinal cord.
Conley is one of a handful of patients selected out of hundreds of applicants with amyotrophic lateral sclerosis (ALS) to be part of the first clinical trial in the country to focus on the safety of injecting human stem cells directly into the spinal cord as a possible treatment for ALS, also known as Lou Gehrig’s disease.
An expectant grandfather, Conley considers this experimental surgery a gift—not just to himself and his family, on the chance that it might slow the progression of his disease, but to others with neurological diseases who might be helped by advances in stem cell therapies.
“Patients like Mr. Conley are agreeing to participate without promise of any benefit. They are doing this to help move science forward,” says ALS Center nurse Meraida Polak.
Two years ago, Conley, from Jackson County, Georgia, started experiencing cramps and twitches in his muscles. Although in good physical shape, he began stumbling over things and falling several times a day. He was diagnosed with ALS, a fatal neuromuscular disease. “It just comes on out of the blue, and people start to get weak. They could develop problems with breathing, chewing, swallowing, even speaking,” says neurologist Jonathan Glass, director of Emory’s ALS Center and principal investigator of the trial site.
While this trial tests only whether this treatment is safe, future trials will determine the treatment’s effectiveness. ALS affects nerve cells in the brain and spinal cord that control muscle movement; patients usually die within two to five years of diagnosis. About thirty thousand people in the US have the condition and nearly seven thousand are diagnosed each year. No cure exists, and many ALS patient advocacy groups and researchers believe stem cell transplants are the best hope for a therapeutic advance.
But stem cell trials and treatments remain highly controversial. Opponents of this research, including groups such as antiabortion advocates and the Catholic Church, want it to be severely restricted or banned. Proponents, including many medical researchers and patient advocacy groups, say that the embryos (often from fertility or abortion clinics) would be discarded and that stem cell research could save countless lives. President Barack Obama signed an executive order last year lifting restrictions on federal funding for embryonic stem cell research. The Georgia Senate approved legislation that would have shut down most forms of embryonic stem cell research in the state, but the proposal failed in the House. The ALS trial at Emory uses fetal neural stem cells, not embryonic stem cells.
On October 20, 2010, Emory neurosurgeon Nicholas Boulis, who developed the technique to deliver the stem cells, and his team carefully exposed Conley’s spinal cord and delivered five injections—each containing about 100,000 neural stem cells—directly into the bottom of his cord. The spinal cord is extremely delicate, says Boulis, and has low tolerance for any form of manipulation. An associate professor of neurosurgery, he practiced the technique for months on the spinal cords of pigs, which are very similar to the cords of humans.
“We’re in uncharted territory here,” he said, of the surgeries performed on ALS patients. “But I am confident this study is taking therapies for the spinal cord to a new level.”
Motor neuron cells begin to die off in individuals with ALS, and the spinal cord isn’t able to send messages to muscles, which causes them to atrophy.
“What I’m hoping for is that these neural stem cells will protect the cells that are still there and possibly even allow the sick cells to reconnect with the muscles,” Glass says. “We want to keep those muscles moving.”
Conley was selected for the surgery in part because he could still walk. The six participants who had their surgeries before him had progressed beyond that level of disease before their stem cell injections. Researchers began the trial with ALS patients who had severe disability (so were at a lower risk for added weakness that might occur as a consequence). They are now moving forward with less disabled patients.
The trial participants are being watched closely to see how they tolerate the surgery, the stem cells, and the antirejection medications.
“We have completed eleven patient surgeries, and all are doing well as of this date,” Glass says. “Stopping the disease in its tracks would obviously be the best outcome, but that is a bit of a utopian thought right now. This is just the starting point.”